There is a huge increase in demand worldwide, particularly for advanced water analytical solutions. The underlying trend is the increasing scarcity of water and the intensifying competition between different water uses. In addition to agriculture and households, it is industry in particular that is steadily increasing its water consumption. Based on this trend, the demand for state-of-the-art analytical solutions for monitoring and controlling water is increasing. HORIBA Tocadero strives for a leading position in the field of advanced water analytics, focusing on both industrial process analytics and environmental monitoring.
Frequently Asked Questions
The total carbon content
- The carbon content in water
- The total organic carbon (TOC)
- Where is the TOC interesting?
- What are typical TOC values?
- Determination of TOC using the difference method
- Determination of TOC using the direct method
- Determination of TOC using the addition method
- What influence does the matrix have on the TOC measurement?
- Technology comparison for TOC determination
Facts about water
Water, our most precious commodity!
The human body consists largely of water. It is vital to drink enough water every day. It is not for nothing that water is considered our most precious commodity. The effects of water shortages or contaminated water have a direct impact on people and the environment. But clean drinking water is finite - a look at the amount available shows this.
A good 71% of the earth is covered by water. Only 3.5% of the world's water is freshwater, half of which is trapped at the poles, in glaciers and permafrost soils and is therefore unusable. A good 2.1 billion people have no or only limited access to clean water. At the same time, industrial water consumption is increasing by 1.9% annually. Many people are not aware of how easily our water is polluted. This is a calculation that will not work out in the long term without sustainable usage concepts and functioning water treatment. This is a responsibility that concerns each and every one of us!

Impurities in water
Water can be contaminated quickly and easily. Many factors deteriorate the quality and have the potential to turn clean drinking water into contaminated and unhealthy food. These can be heavy metals, microbial contamination or harmful organic and inorganic compounds. Gastrointestinal complaints, kidney damage or damage to the nervous system are just a few examples of possible consequences.
Many sources can be found in our own household. Old pipe materials containing lead lead to heavy metal contamination and quickly lead to the limit values being exceeded. Microbial contamination should not be neglected either. The best-known examples are legionella and pseudomonads. Modern systems heat the water at regular intervals and kill the pathogens. Lack of use and stagnation of the water flow also lead to increased contamination. Pathogens multiply undisturbed and form harmful wet germs. These cause serious illnesses such as pneumonia or urinary tract infections.
We dispose of faeces and some substances that do not belong in wastewater via municipal wastewater. Wastewater treatment plants treat the contaminated water. It is discharged into the municipal water network or the environment at drinking water quality. One of the biggest users of water is industry. Water is used for the manufacture of products, for cooling and heating processes or as a solvent. In each of these steps, contaminants from the process can enter the wastewater system in the event of a leak or accident. These processes are therefore closely monitored and contaminated water is collected for post-treatment.
Parameters in water analytics
In water analytics, a distinction is made between physical and chemical properties and ingredients. Individual samples are measured in laboratories using wet-chemical and physical measurement methods. Large processes or water flows are monitored using measuring probes or online analyzers. Control stations detect changes in the water composition and react directly to the respective situation. The most important physical parameters are the pH value, conductivity, UV absorption and coloration.
pH value: According to the Drinking Water Ordinance, the pH value should be in the range of 6.5 to 9.5. It is a measure of the acidic or alkaline character.
Conductivity: Salts and minerals have an influence on the electrical conductivity of water. The higher the content, the greater the conductivity. However, it is not possible to draw conclusions about suitability for drinking water.
UV absorption: Many organic substances absorb light of specific wavelengths in dissolved form. UV absorption can therefore be used as a rough indicator of organic impurities.
Coloring: Similar to UV absorption, coloring provides an initial indication of possible contaminants. These can be organic or inorganic in nature. However, the analysis is in the light spectrum visible to humans.

Individual chemical substance groups are often determined as sum parameters - i.e. summarized in one value. A comparison can be made with a bag of sweets. We have chocolate, wine gums and fizzy sweets, but we only count the number of bags we still have in the cupboard. One of the most important sum parameters is the total organic carbon content TOC. The chemical oxygen demand, the total nitrogen content or biological oxygen demand are other interesting parameters. In addition to the sum parameters, individual chemical parameters provide a more specific conclusion. The most important are:
Carbonat: The carbonate ions (CO3-) have a direct influence as a measure of water hardness. When water is heated, lime precipitates in the form of calcium carbonate (CaCO3). Water that is too soft can damage pipes, water that is too hard leads to calcification.
Ammonium, nitrate and nitrite: These three ions indicate the content of bound nitrogen. The ratio provides information about the prevailing electrochemical conditions. This is also influenced by bacteria and fungi, which thrive in different environments. High ammonium levels often indicate hygienically questionable contamination. The main sources of nitrates are fertilizers, wastewater and landfill leachate. Nitrites indicate rotting and decaying processes.
Sulphate, sulphite and sulphide: Sulphate, sulphite and sulphide: These sulphur compounds often have a geological origin, e.g. in gypsum-containing soils or sediment deposits. As a "lead ion", sulphate is a measure of leaching from building rubble. It can lead to the formation of hydrogen sulphide - a colorless gas with a characteristic stench of rotten eggs.
Chloride: In groundwater, chloride usually has a geological origin. Excessively high values indicate fertilization or winter salt spreading.

Phosphate: Phosphates are also dissolved from soil layers. The application of liquid manure or artificial fertilizers increases the values. Elevated values in local wastewater sections indicate defective pipelines, as detergent is released from the wastewater into the environment.
Heavy metals: Individual heavy metals such as lead, arsenic, strontium, copper or uranium are also determined individually if required. They provide information on old water pipes, geological origin or environmental disasters such as the Chernobyl incident. However, "metals" are not always bad - trace elements such as iron, copper and zinc are very important for our bodies.
Water treatment
The water quality used depends heavily on the area of application. Choosing the right treatment is essential and adapted to the requirements. Municipal wastewater is brought to drinking water quality in wastewater treatment plants through several purification steps. The treatment plant removes particles and suspended solids and treats the water chemically and biologically.
Even stricter requirements are placed on water in the pharmaceutical industry. Described as ultrapure water, it contains almost no impurities. There are many ways to achieve this. Processes such as distillation, microfiltration, reverse osmosis or the use of ion exchangers are just some of them. Combinations of different techniques are often used.
For the cooling of chemical processes, however, the use of ultrapure water is beyond the scope. The costs are not in proportion to the benefits.
Water in industry / Virtual water
We use water every day for washing, cooking, cleaning and drinking. On average, every German consumes around 130 liters a day. However, this is only the water that we come into direct contact with every day. The actual figure is much higher. Every object consumes water during the manufacturing process. 1 kg of paper requires 5 to 6 times the average daily water consumption of a German citizen. This water is also known as virtual water.
But what is it used for? Depending on the product, water is used for different purposes. The most obvious are: Water as a solvent for chemical processes and for the dilution and production of food and pharmaceuticals. Other areas of application, the scope of which should not be underestimated, are: for cooling and temperature control of processes or for cleaning.
Our products for TOC analysis
The Tocadero ONE is a modern, low-maintenance TOC analyzer that combines maximum precision, efficiency, and user-friendliness. High-temperature combustion at 1,200 °C without a catalyst enables complete oxidation of even stable carbon compounds—directly in the process, in real time, and under the most demanding conditions. Developed as a flexible yet highly precise online analyzer, it is used in many areas. Also available in an Ex version.
The
Tocadero EVO is a versatile TOC measuring device that has been specially developed for monitoring organic contamination in ultrapure and pure water. With its innovative
UV oxidation and differential conductivity measurement, this TOC analyzer is ideal for the pharmaceutical industry, microelectronics, power plants, electrolysis, and chemical production.
The total carbon content
The carbon content in water - TC
The total carbon content ("total carbon", TC) is a measure of the quality of the water and is made up of the organic ("total organic carbon", TOC) and inorganic ("total inorganic carbon", TIC) components. Both parameters can be determined using differential measurement methods or individual measurements. TOC is also divided into its solid ("non purgeable organic carbon", NPOC) and volatile components ("purgeable/volatile organic carbon", POC/VOC). The NPOC consists of the dissolved components ("non purgeable dissolved carbon", DOC) and the non-dissolved particles.

The total organic carbon content - TOC
The abbreviation TOC stands for "total organic carbon". TOC describes the contamination of water with organic substances and is one of the most important parameters for water quality. As a sum parameter and absolute value, it is not substance-specific and reflects the quantity of carbon atoms contained in the water. Depending on the type of water, e.g. whether ultrapure or waste water, the concentration ranges from a few ppm to several 10,000 ppm. Thermal oxidation has proven to be the method of choice. The carbon is converted to CO2, which can be easily determined using UV absorption methods.
It is standardized internationally by DIN EN 1484 and ISO 8245 and is therefore gaining increasing international acceptance. Unlike chemical oxygen demand, TOC is relatively easy to determine using online measurement methods. DIN EN 1484 describes three different methods for determining TOC. In most cases, the difference method is used. In two individual measurements, the difference between the total carbon content TC and the inorganic carbon content TIC is calculated.
Where is the TOC interesting?
The TOC is a sum parameter that tells a lot about the water quality. It is therefore well suited for monitoring and controlling water treatment processes. It helps to comply with limit values, detect leaks and optimize existing processes. The most common applications are in the areas of drinking water, process water and waste water. The TOC content is also used to monitor the treatment of water for processes in the chemical industry. Consistent water quality is ensured and the discharge of polluted water into inland waters is prevented. In the case of large dischargers, the inflows to the wastewater treatment plant are often monitored, as the wastewater treatment plant could tip over in the event of an accident, for example.
What are typical TOC values?
The organic carbon content varies greatly depending on the type and origin of the water. Treated water, such as that used in the pharmaceutical industry, tends to have a very low content of a few ppb. Even our drinking water only has a low content of 100 ppb to 10 ppm. Process water or waste water, on the other hand, has a very high proportion of TOC and must inevitably be purified.
| Type of water | Typical TOC (range) | Typical TOC present as particles |
|---|---|---|
| Bog | 33 ppm (10 to 60) | 4 ppm |
| Marsh | 17 ppm (10 to 60) | 3 ppm |
| Eutropic lake | 12 ppm | 3 ppm |
| Oligotrophic lake | 2,2 ppm | 0,2 ppm |
| River | 7,0 ppm ( 1 to 10) | 2,5 ppm |
| Precipitation | 1,1 ppm | 0,1 ppm |
| Ground water | 700 ppb | |
| Sea water | 500 ppb | 50 ppb |
| Waste water | up to 1.000 ppm | |
| Process water | application-specific (e.g. >10.000 ppm) | |
| Drinking water | 100 ppb to 10 ppm | |
| Purified water | 1 ppb to 500 ppb |
Determination of TOC using the difference method
The differential method has established itself as the method of choice for high TOC concentrations, for example in the field of municipal wastewater. In the first step, the total carbon content TC is determined. In a second measurement, the inorganic components TIC are measured. If these are subtracted from the TC, the TOC value is obtained (TC-TIC = TOC).
This is the method of choice, especially in the presence of volatile organic carbon, as it provides the most accurate results. Although the POC is stripped out together with the TIC, it is not detected by the detector. At high TOC concentrations, it provides the best reproducibility and the most accurate results.
Determination of TOC using the direct method
If the TOC content is very low compared to the TIC, the direct method is the method of choice. It is usually used for drinking water and ultrapure water. In this case, the TOC is determined as NPOC. The sample is acidified and the inorganic components are converted to CO2 and finally stripped out. What remains are the organic components, which are then determined directly. The volatile components are not taken into account, which is why the NPOC is also referred to as non purgeable organic carbon (NPOC). This method is not only suitable for low TOC contents, it is also used for low POC contents.
Determination of TOC using the addition method
The addition method is always used when the volatile organic components of the TOC must not be neglected. Especially with high concentrations of POC, the direct method leads to incorrect results. To avoid this, the POC is determined in addition to the NPOC. For this purpose, all volatile organic components (POC) are stripped out. The CO2 contained is bound to an absorber. The remaining volatile organic compounds are oxidized and the resulting CO2 is determined directly using an NDIR. The POC value is then added to the NPOC.
What influence does the matrix have on the TOC measurement?
The type of sample has a major influence on the reproducibility and accuracy of TOC measurements. Particularly with difficult matrices, the correct detection type must be selected and the TOC measuring device adapted to the respective conditions. Large particles can block the flow line or not be completely digested. This results in lower results. This can be avoided by using thermal methods, which break down the entire sample including particles. Strongly acidic or alkaline sample flows can damage the device and lead to total failure. Choosing the right materials for the flow line is crucial. If the device is used in an explosion-proof area, it must be certified in accordance with the currently applicable standards.
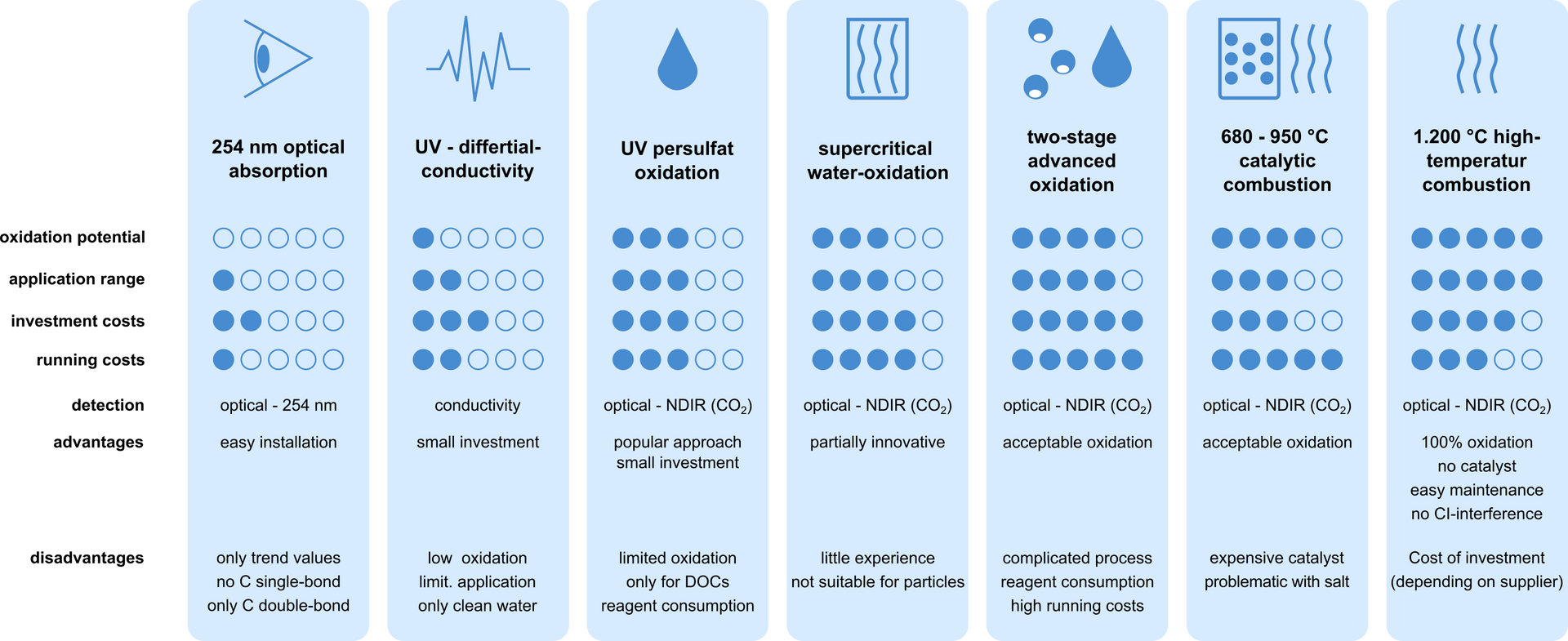
Technology comparison for TOC determination
Various technologies for TOC determination have become established on the market, each of which has advantages and disadvantages depending on the area of application. Almost all methods convert the existing TC to CO2, which is detected using optical methods. The simplest technique is the direct measurement of adsorption. As the most cost-effective option, it provides the least information, as no C single bonds and only trend values are detected. Other methods such as UV persulphate oxidation only have a limited oxidation potential. This can result in lower results. Processes that use catalysts or two-stage advanced oxidation have high operating costs that must be factored in from the outset. The issue of maintenance also plays a decisive role in online measurement technology. High-temperature combustion at 1200°C, on the other hand, offers a number of advantages. Due to the high degree of oxidation, even small particles are 100% converted, which provides reliable results. If only a few moving parts are installed, the maintenance effort is also reduced to a minimum. Only monthly visual inspections and annual maintenance are required. The operating costs and the monthly time required are reduced to a minimum.
The total nitrogen content TNb
TNb as a sum parameter
As a sum parameter, TNb records the total content of bound nitrogen in a sample. Its determination is standardized in DIN EN 12260:2003. Combined in one device, simultaneous TOC and TNb measurement saves time and eliminates the need for a second sample preparation. The method can be used for the analysis of surface water, waste water and sewage treatment plant effluents. Nitrogen compounds such as nitrates, nitrites, ammonia, ammonium or organic nitrogen compounds are detected. The TNb thus provides information about the nutrient content in the water. If the input is too high, e.g. due to excessive agricultural fertilization, problems can quickly arise.
Detection methods of nitrogen
In water analysis, the nitrogen content is determined using chemiluminescence or electrochemical detection. Both methods are identical in their accuracy; dissolved elemental nitrogen is not detected. Electrochemical detection is very low-maintenance and requires no additional equipment, which is why it is often the method of choice. It can also be combined with high-temperature online TOC devices. To determine nitrogen using chemiluminescence detection, all the nitrogen is converted to nitrogen oxide, which then reacts with ozone. The process is more complicated to implement and requires additional measures with regard to occupational safety.




